Overview and History of Parabolan
Parabolan is the brand name and trade name for the anabolic steroid Trenbolone Hexahydrobenzylcarbonate, commonly and informally abbreviated as Tren Hex or Trenbolone Hex. It is a longer acting esterified variant of the anabolic steroid Trenbolone, which is the most popular in the form of Trenbolone Acetate (a much faster acting esterified variant of Trenbolone). The Hexahydrobenzylcarbonate ester extends Trenbolone’s half-life to the extent of approximately 14 days rather than 3 days, which is the half-life of Trenbolone Acetate. Trenbolone in both formats saw very brief periods of use as an approved medical drug for human use, as Trenbolone Acetate was marketed for a brief time during the 1980s as Finajet and Finaject before being pulled from the market and disapproved for human use circa 1987. Parabolan was deemed more suitable for human use during its run on the prescription drug market due to its longer acting and slower releasing properties than Trenbolone Acetate, making it much more suitable and comfortable for clinical and medical use with less frequent injections required as a result. Trenbolone itself, no matter the ester attached to it, is known as the most powerful conventionally available anabolic steroid in use by athletes and bodybuilders today.
Trenbolone itself was first unveiled, tested, and published in 1967 by Roussel-UCLAF[1]. At this time, Trenbolone Acetate was unveiled, which was the prototypical fast acting Trenbolone ester, as well as Trenbolone Undecanoate, which was the very first long acting Trenbolone ester. Trenbolone Hexahydrobenzylcarbonate was created in France as a French variant of a long-acting Trenbolone compound. The hexahydrobenzylcarbonate ester granted Trenbolone almost the exact same half-life as the decanoate ester, as they were very similar. Trenbolone Hexahydrobenzylcarbonate was then picked up by Negma Laboratories in France, which then marketed Tren Hex under the trade name Parabolan on the prescription drug market. It was also sold under the name of Hexabolan, which was lesser known at the time. Trenbolone in the form of Trenbolone Hexahydrobenzylcarbonate (sold as Parabolan) was the only variant of Trenbolone that was officially manufactured and approved for human use. Although Trenbolone Acetate was briefly marketed as Finajet and Finaject for human use, its run as a human grade medicine was very short and brief. Trenbolone Acetate was and is today only utilized as a veterinary drug.
During Parabolan’s reign as a human-approved medicine, it was utilized primarily in France as a drug for the treatment of muscle wasting, muscle wasting diseases, malnutrition, and as a drug to treat various forms of osteoporosis. Because of the extremely strong androgenic nature of Parabolan (approximately 5 times the androgenic strength of Testosterone), there were issues administering it to children (specifically, female children). Its use in other traditionally androgen-sensitive patient types (such as the elderly and adult females) was not as troublesome. Parabolan was very successful on the French prescription drug market for a long while, but in 1997 it was intentionally discontinued by Negma. This was primarily due to the issue of increased anti-steroid hysteria and sentiment during the late 1980s and early 1990s that prompted many pharmaceutical companies to completely discontinue and eliminate the production of various anabolic steroids (especially those anabolic steroids that were utilized more by athletes than for medical purposes. Unfortunately, Parabolan was one of the anabolic steroids that had succumbed to this fate. Fortunately, however, an extremely small amount of pharmaceutical Parabolan is still manufactured today (though not in France), and it is an extremely rare product to the point where it might not even be considered worth going through the hassle to obtain it, as counterfeit Parabolan is likely in circulation in greater numbers than legitimate pharmaceutical grade Parabolan.
Trenbolone in general is widely regarded as being currently unapproved for human use by the medical establishment. However, the good news is that it is under current investigation in clinical studies as a more selective anabolic steroid than others, giving it the potential for medical application[2]. This could be a very strong indication that Trenbolone might soon experience re-entry into the human prescription drug market and re-approval for human use, but it is unknown how long it might be before this happens.
Chemical Characteristics of Parabolan
Trenbolone itself is a synthetic derivative of Nandrolone, meaning it is a modification of Nandrolone. This also means that Trenbolone is classified as a 19-nor compound, meaning it lacks the 19th carbon that is typically held by Testosterone and all other anabolic steroids (except for Nandrolone). Both Nandrolone and Trenbolone do not possess this 19th carbon, and they are therefore classified as 19-nor compounds. This also results in Trenbolone and Nandrolone being categorized as Progestins, meaning they exhibit Progestogenic activity within the body, and will exhibit varying degrees of interaction with the Progestosterone receptor.
Trenbolone in particular possesses double bonds at carbons 9 and 11 due to the fact that the hydrogen atoms that previously occupied the free electrons on these carbons were removed. Therefore, double-bonds between those carbon atoms and their neighboring carbon atoms had to be established to fill in the void. These modifications create three distinct characteristics and abilities for Trenbolone:
1. A significantly larger affinity for the androgen receptor is created, resulting in Trenbolone being much more potent than its parent hormone Nandrolone[3]. Trenbolone possesses 5 times the anabolic muscle building strength than Testosterone, with Testosterone’s anabolic rating being 100 and Trenbolone’s anabolic rating being 500.
2. Trenbolone becomes completely resistant to aromatization (the conversion into Estrogen), as the aromatase enzyme (the enzyme responsible for aromatization) cannot recognize Trenbolone as an appropriate substrate for chemical reaction[4]. This is different from Nandrolone, which is merely more resistant to aromatization than Testosterone, rather than completely immune to it like Trenbolone is.
3. A very high resistance to metabolism in the body. These modifications allow Trenbolone to linger around in its active form longer than most other anabolic steroids, and this is also evident by the fact that the majority of Trenbolone excreted in the urine is in its original format rather than a greater amount being excreted as metabolites. This also owes to its greater anabolic effect.
Parabolan in particular is a modified variant of Trenbolone via the addition of a hexahydrobenzylcarbonate ester onto the 17-beta hydroxyl group on the steroid structure of Trenbolone. This results in a slower rate of release and a longer half-life of the compound, which is approximately 14 days. The body must work to first break off this ester bond before the hormone can be used by the body, and this is why a longer half-life is experienced. The addition of an ester to the hormone merely serves to augment the half-life of the hormone, and does not alter the pharmacological effects of Trenbolone in the body.
Properties of Parabolan
Parabolan is an extremely versatile anabolic steroid available to athletes and bodybuilders that can be literally utilized for any purpose desired. It is an extremely potent anabolic steroid that does not require high doses in order to elicit favorable performance or physique changes. It is 5 times the anabolic strength of Testosterone, making it an ideal choice for bulking, strength gaining, and/or lean mass addition. The added benefit of Parabolan is the inability for it to be aromatized into Estrogen, resulting in absolutely no water retention or bloating from Parabolan alone. The result is a complete lack of Estrogen related side effects, allowing the user to completely avoid the puffy and bloated look that heavy aromatizable androgens (such as Dianabol) will provide. The result is considerable and dramatic lean mass and strength gain with Parabolan when combined with an appropriate diet and training regimen.
Of course, Parabolan can also be easily utilized for the purpose of fat loss, cutting, and pre-contest. It is in fact the most highly sought after anabolic steroid by competitive bodybuilders, and is considered such an indispensable addition in a competitive bodybuilder’s cycle that it is commonly referred to as “the nectar of the Gods”. Trenbolone’s extremely strong nutrient partitioning effects can result in fairly rapid fat loss when combined with a clean caloric deficit and proper training. Parabolan’s highly androgenic nature also tends to bring out the hardness and vascularity of the muscles and physique, creating the “3D” look commonly aspired by bodybuilders (provided that body fat is low enough to see this).
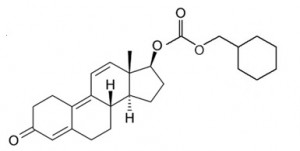 Trenbolone Hexahydrobenzylcarbonate (AKA Parabolan)
Trenbolone Hexahydrobenzylcarbonate (AKA Parabolan)
Chemical Name: 17beta-Hydroxyestra-4,9,11-trien-3-one
Molecular Weight: 410.55 g/mol
Formula: C26H34O4
Original Manufacturer: Negma Laboratories
Half Life: 14 days
Detection Time: 4 – 5 months
Anabolic Rating: 500
Androgenic Rating: 500
Parabolan References:
[1] J. Mathieu, Proc.lntern. Symp. Drug Res. 1967, p 134. Chem.lnst. Can., Montreal, Canada.
[2] Tissue selectivity and potential clinical applications of Trenbolone (17beta-hydroxyestra-4,9,11-trien-3-one): A potent anabolic steroid with reduced androgenic and estrogenic activity. Yarrow JF, McCoy SC, Borst SE. steroids. 2010 Jun;75(6):377-89. doi: 10.1016/j.steroids.2010.01.019. Epub 2010 Feb 4.
[3] Characterisation of the affinity of different anabolics and synthetic hormones to the human androgen receptor, human sex hormone binding globulin and to the bovine progestin receptor. Bauer, Meyer et al. Acta Pathol Microbiol Imunol Scand Suppl 108 (2000):838-46.
[4] Unique steroid congeners for receptor studies. Ojasoo, Raynaud. Cancer Research 38 (1978):4186-98.