Low Testosterone and Endogenous Testosterone
As previously explained throughout this article, the term ‘endogenous’ refers to anything manufactured and secreted from within the body, while the term ‘exogenous’ refers to anything introduced into the body from an outside source (via ingestion or injection, for example). When the phrase ‘endogenous Testosterone’ or ‘natural endogenous Testosterone production’ or any variation thereof is used, it is referring to the body’s own production and secretion of Testosterone, which occurs in the Leydig cells of the testes. Exogenous Testosterone, is, of course, administered through various routes of administration such as transdermal administration, injection, or ingestion via oral tablets or capsules. Within this section of this Testosterone article, the concern is with the endogenous Testosterone production, the dynamics surrounding it, and perhaps the most important and most concerning and examined aspect in society today: low Testosterone. This subsection will examine the risks of low Testosterone, the causes of low Testosterone, the typical treatments for low Testosterone, and the considerations for those looking to engage in long term Testosterone replacement therapy (TRT) as one of the treatments for low Testosterone.
A Brief Description of Endogenous Testosterone
It has previously been described in-depth at the beginning this article of how Testosterone, whether endogenously or exogenously, operates at the cellular and systemic level in the human body. The manufacture and secretion of endogenous Testosterone by the Leydig cells of the testes is but only one of several axis points in a larger axis network known as the HPTA (Hypothalamic Pituitary Testicular Axis). This is a component of the endocrine system that is composed of various hormones that act as messengers to continue a signaling cascade through each axis, which will ultimately lead to the production of Testosterone. This endogenous Testosterone production is the final product that the majority of individuals are concerned with, and the other hormones that are involved in the operation of the HPTA serve as intermediary (but very necessary) hormones in the functioning of endogenous Testosterone production. The understanding of endogenous Testosterone production and how the HPTA works to enable the manufacture of Testosterone is essential to the overall understanding of how the body secretes endogenous Testosterone, the causes of low Testosterone, how it can be remedied, as well as the issue of post cycle therapy (PCT) following any anabolic steroid cycle, and any other specific details in regards to this aspect of the endocrine system.
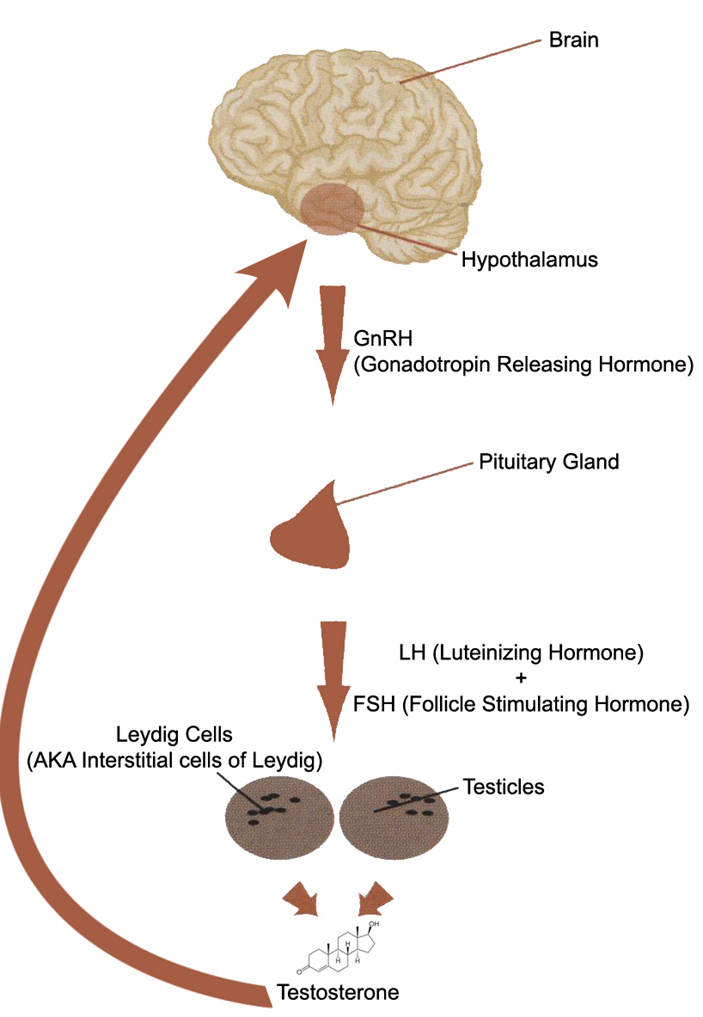
The diagram displayed above is a picture of the HPTA. The HPTA, as previously described, is a system of interconnected axes that regulates and determines endogenous Testosterone secretion, as well as how much Testosterone is determined to be circulating throughout the body at any given time. How much maximum endogenous Testosterone is manufactured and circulating within each human body is predetermined by a set of ‘blueprints’ and ‘instructions’ in the form of their genetics (DNA). There are indeed other determining factors that also influence the amount of Testosterone any person will endogenously secrete as well. These other factors include: age, diet, body composition, lifestyle habits, and physical activity. Every single one of these factors, in addition to the absolute prime determining factor of genetics (DNA), is the ultimate arbiter in determining how much endogenous Testosterone is manufactured.
The HPTA operates through a system dynamic known as negative feedback. This is specifically known as the negative feedback loop, which is a self-regulating mechanism of a system. This negative feedback loop will influence the operation of the HPTA in such a way so as to reduce changes. What this means is that the body will decrease its endogenous Testosterone production if what it deems as excessive levels of Testosterone are detected circulating through the bloodstream. The HPTA will then adjust the output signals accordingly if excessive (or insufficient) amounts of Testosterone are detected. This recognition and alteration process is the basic description of the operation of the negative feedback loop. This whole process is controlled by the hypothalamus, which serves as the ‘master’ endocrine gland for literally all endocrine and hormonal processes in the body. What causes the hypothalamus to determine whether there is ‘too much’ or ‘too little’ Testosterone in circulation is determined by the genetic code (DNA) as mentioned earlier. This negative feedback loop is one of the many (but major) processes involved in the body’s maintenance of hormonal homeostasis. Homeostasis is defined as the regulation of a system, and this can be the regulation of a computer system, a mechanical system, or an ecological system. In this particular case, it is referring to the internal systems of the body – specifically, the endocrine system. This is achieved by the body for the purpose of maintaining steady and continuous satisfactory conditions. Every endocrine axis and every endocrine gland within each axis operates by way of the negative feedback loop in one manner or another, and to fluctuating degrees.
The HPTA consists of and operates by way of the following 5 hormones in order to maintain homeostasis:
– GnRH (Gonadotropin Releasing Hormone)
– LH (Luteinizing Hormone)
– FSH (Follicle Stimulating Hormone)
– Testosterone
The first axis point within the HPTA is the hypothalamus. The hypothalamus will, through monitoring of the bloodstream (as the contents of blood plasma flows through the veins and capillaries passing through it), detect a necessity for the body to produce more endogenous Testosterone, and in response it will release variable amounts of GnRH. GnRH is a signaling hormone that will act to inform the next axis point, the pituitary gland, to commence the production and secretion of two other important signaling hormones known as gonadotropins: LH (Luteinizing Hormone) and FSH (Follicle Stimulating Hormone). LH and FSH are the two hormones that exert the signaling process to the third axis point, which is the testes, to begin the synthesis and secretion of Testosterone. This is the final stage, and ultimately the final manufacture and production goal of endogenous Testosterone, in the HPTA.
The inhibition, reduction, suppression, and/or complete shutdown of the HPTA (and subsequently, endogenous Testosterone production) is essentially determined by two primary hormones:
– Excess Testosterone
– Excess Estrogen
There are, of course, other conditions and hormones that might act to impede and subdue HPTA function, such as physical trauma to the testes, or other hormones and hormonal factors such as Progestins and Prolactin. However, when the concern is with the normal feedback loop and how the body’s own regulatory systems serve to inhibit (or stimulate) endogenous Testosterone production, the two primary hormones that are determinant here are Testoserone and Estrogen. When the hypothalamus identifies surplus amounts of Testosterone and/or Estrogen in the bloodstream, the hypothalamus will work to reinstate homeostasis by engaging in the opposite process of what was previously outlined – it will suppress or shut down its manufacture and release of the various signaling hormones throughout the axis. Excesses of Testosterone or Estrogen that can signal this negative feedback loop can result either from the use of exogenous androgens on an anabolic steroid cycle or even from endogenous imbalances within the body. There are many different factors that can affect this process.
When the hypothalamus will reduce or stop its production of signaling hormones within the axis, the opposite process essentially results: the hypothalamus will halt the release of GnRH, which then halts production of LH and FSH, which then ultimately reduces or halts production of Testosterone at the Leydig cells of the testes. It is only once the hypothalamus’ ideal hormonal homeostasis (as determined by genetics and the other factors described) has been restored, only then will the secretion and release of the various signaling hormones within the HPTA will begin once again. Such a process can require months before the body will restore its function to that of normal levels again, and this is without the intervention of any exogenous Testosterone stimulating compounds. What determines how long this will be achieved by the body all on its own capabilities is dependent on many factors – how long the suppression has been for, the individual’s personal genetics, environmental factors, and many more.
Signs, Symptoms, and Risks of Low Testosterone
The awareness of low Testosterone and its risks and impairments has increased dramatically over the recent decade or two at the time of this writing (2013). There is an increasing amount of medical professionals and physicians that have found themselves treating more and more male patients concerned with and exhibiting signs and symptoms of low Testosterone, which is a diagnosis that is becoming increasingly common as the years go by. This is likely due to the fact that more and more males are becoming increasingly aware of the condition rather than an increase in the incidence or development of the condition. It is a normal aspect of aging that Testosterone levels in males decline as they grow older.
There are two primary categories for the causes and conditions of low Testosterone, both of which have been mentioned thus far in this all-inclusive Testosterone article. They are andropause and hypogonadism. Andropause is defined as the condition descriptive of males (mostly middle-aged males and older) suffering from age-related decline in proper endogenous Testosterone production. Hypogonadism is defined as a condition in which the testes are manufacturing inadequate and insufficient endogenous Testosterone in individuals of any age and could be due to any number of a broad variety of causes such as genetics, physical injury, disease, or any other reason. Andropause is a form of hypogonadism, as it is a hypogonadal condition. However, andropause is a subcategory that refers specifically to age-related decline of Testosterone.
It is a very well documented fact that as males age, serum Testosterone levels will decline. What is interesting to note, however, is that in many cases, LH levels will for the most part remain unchanged while Testosterone levels decline and low Testosterone is associated with negative alterations in body composition, energy levels, muscle strength and mass, sexual, physical, and cognitive functions as well as mood[1]. The same studies that have determined this have also determined that the age-related low Testosterone in the presence of increasing or unchanged serum Testosterone levels demonstrates that this is a result of the aging of the Leydig cells of the testes. The Leydig cells have undergone, due to the aging process, desensitization to LH stimulation whereby now their response to LH has diminished substantially. This is one such cause for low Testosterone in those that suffer from andropause, and is perhaps the most common cause. It is also one of the causes of which various treatments for this particular cause of low Testosterone will not work (this will be explained shortly). In any case, it is very evident that the frailty commonly displayed by aging men is resultant of these declines in endogenously manufactured androgens, as it is well known that this decline in Testosterone is a key contributor to sarcopaenia (loss of muscle mass and strength), which is a key sign of increased frailty whereby TRT (Testosterone replacement therapy) has been suggested as a treatment for this[2]
These signs and symptoms of low Testosterone will manifest themselves regardless of the cause, whether it is andropause or general hypogonadism. As mentioned above, the symptoms of low Testosterone include the following:
– Sexual dysfunction (loss of sex drive and libido, erectile dysfunction, fewer erections)
– Fatigue
– Thinning of the skin
– Loss of muscle mass and strength (sarcopaenia)
– Increase in body fat
– Negative mood changes (increase in depression and negative thoughts)
– Low motivation
– Low energy
– Emotional fluctuations
The health complications and risks resulting from low Testosterone can be quite concerning as well. It goes without saying that the frailty issues and all of the signs and symptoms listed thus far could even be considered risks and health complications in and of themselves, but there have been various well documented examples of health risks of low Testosterone. These include anemia[3], chronic fatigue[4], development of osteoporosis[5], development of diabetes mellitus[6] [7], cardiovascular disease and chronic heart failure[8], and mental/psychological problems including depression[9] as well as the beginnings of evidence for the development of dementia[10].
It is interesting to note, however, that the treatments for low Testosterone, particularly andropause, are a conflicted one. Individuals that exhibit general hypogonadism as a result of various other causes are far more likely to receive surefire treatment for their condition as opposed to age-related low Testosterone (andropause) even though andropause is becoming an increasingly common condition. It is as of yet not fully recognized by all medical professionals as a valid disorder warranting treatment, although trends point towards the recognition and treatment of the condition to becoming more popularly accepted and widespread. In any case, the acceptance of the condition of andropause and its treatment is currently more widely accepted in places outside of the United States, such as Australia and Europe than it is within the United States[11].
In any case, a thorough examination of the patient is always necessary when determining low Testosterone. Physicians will want to rule out any possible other explanations for the various symptoms described prior to attributing them to low Testosterone. It is imperative that the individual be first aware of the signs and symptoms of low Testosterone, which are normally all-inclusive to varying degrees. Following this, the proper consultation with medical professionals will be necessary involving the proper testing procedures including blood work in order to pinpoint and determine that the symptoms are in fact caused by low Testosterone. Following this, the appropriate treatments can be applied.
Every individual should also remember that TRT is normally not a temporary therapy/treatment, and is normally a lifetime therapy, meaning the consistent administration of Testosterone is for life. Every individual considering TRT must keep this factor in mind.
The long-term safety of Testosterone replacement therapy has been well-documented in all aspects, especially in the examination of prostate health of TRT patients, which has been a general concern among those curious about TRT where it has been determined that the risk of prostate-related problems is no greater in TRT patients than that of the general population[12]
Typical Treatments For Low Testosterone, Monitoring of Vital Markers, and Considerations of Long-Term TRT
The general treatments for all forms of low Testosterone can vary, but they can be generally narrowed down to two primary medical treatments (in no particular order):
1. Testosterone replacement therapy (TRT)
2. HPTA recovery attempts with Testosterone stimulating compounds
Testosterone replacement therapy (TRT): The first listed treatment, TRT, which has already been discussed fairly in-depth throughout this all-inclusive Testosterone profile, is perhaps the most widely utilized treatment, and as such it will be covered first. Although there is no set-in-stone proper protocol of treatment for low Testosterone, TRT is generally the first and foremost treatment that is usually administered by medical professionals. However, it only makes sense that prior to the administration of exogenous Testosterone, an attempt to recover the patient’s endogenous Testosterone production and HPTA function should first be approached. This has become an increasingly popular among low Testosterone treatment protocols among physicians but is not yet popular enough that the majority are adopting this two-stage method of treatment. As it stands, TRT is currently the first line of treatment, and therefore TRT is what will be covered first. The administration of exogenous Testosterone at a dose that mimics the body’s normal physiological range of approximately 50 – 70mg weekly of Testosterone is normally the target. In such a case, the use of a long-estered Testosterone variant such as Testosterone Cypionate or Enanthate administered once weekly at a dose of 100mg per week is sufficient. Once the weight of the ester and any possible wastage is factored out, the resulting amount is normally within the physiological range. Other forms of application such as transdermal gels (AndroGel) normally prescribe a 5 – 10mg daily topical application, which equates to 35 – 70mg weekly.
Of course, any application type can be adjusted according to the patient and physician’s determination and decisions. Generally, this involves the patients input and feeling as to how they feel they are doing during their treatment, followed up with blood work in order to monitor what the blood plasma levels of Testosterone actually are. Blood work during the course of TRT of any sort should involve a consistent monitor of all hormone panels, which should always include at the very least:
– Total Testosterone levels
– Free Testosterone levels
– SHBG (Sex Hormone Binding Globulin)
– Estrogen levels (Estradiol in particular)
– Full thyroid panel (T3, T4, and TSH – Thyroid Stimulating Hormone)
– Liver function (bilirubin, alkaline phosphatase, aspartate aminotransferase – formerly known as SGOT – and alanine aminotransferase – previously known as SGPT)
– Total cholesterol – HDL and LDL
– Prostate Specific Antigen (PSA)
These are generally the most essential blood values to monitor for individuals on TRT. The majority of physicians that understand proper TRT should already have these tests and functions covered. Additional values to be tested for during blood work will normally also be covered, but those listed above are the most essential. Total Testosterone levels are perhaps the most important due to the fact that the patient and physician must understand where the current form of exogenous Testosterone administration is placing their Testosterone levels at, followed by how much of that Testosterone is actually free and how much is bound to SHBG. After this, Estrogen levels are perhaps the second most important factors to monitor, as many individuals, although they are utilizing a TRT dose of Testosterone, can succumb to rising Estrogen levels that might possibly present Estrogenic side effects. In such cases, the use of an aromatase inhibitor at a mild dose might be necessary and/or an adjustment of Testosterone doses to a lower dose. Normally this would be guided under the physician’s recommendations, but most TRT patients can expect to encounter the use of either Aromasin (Exemestane) or Arimidex (Anastrozole) as one of the two aromatase inhibitors utilized at a moderate dose for Estrogen control if aromatization problems arise during TRT. Letrozole (Femara) is not normally utilized due to its impractically strong nature. Following these vital markers, liver function, cholesterol, and PSA levels are important to monitor, as they are all potential values that Testosterone administration can and do indeed influence and change. As such, blood tests should be performed every two months as they all should be monitored at that approximate frequency throughout TRT administration.
One final important note to make in the considerations for TRT is the fact of Leydig cell atrophy of the testes. Although it is understood that TRT is a treatment for life, there are male patients that might perhaps wish to disengage from TRT for any reason at any point in their life. Others might elect to simply wish to avoid the testicular atrophy during TRT administration despite the dedication to long-term lifelong therapy. One of the most common questions asked by TRT patients is “how can I avoid testicular atrophy during Testosterone replacement therapy?” The answer is in the periodic use of HCG (Human Chorionic Gonadotropin). HCG is a gonadotropin derived from the urine of pregnant women, but the interesting thing about it is that it holds a component of its structure that is structurally identical to LH (Luteinizing Hormone). As such, it will act in an identical manner to LH in the human body at the testes in men, and thus stimulate the Leydig cells to begin or continue the production of Testosterone, thereby avoiding prolonged periods of Leydig cell and testicular atrophy. Although there are a myriad of different protocols for the periodic administration of HCG during TRT, the general concept is to administer HCG every so often (approximately once every several weeks to once every two months) at approximately 500 – 1000iu every two to three days for a total period of 1 – 2 weeks so as to maintain testicular size and function.
2. HPTA recovery attempts with Testosterone stimulating compounds: Although a lesser known and lesser attempted treatment, it really should be utilized more frequently and prior to the resort of utilizing exogenous Testosterone for TRT for the rest of the patient’s life. It has been previously mentioned, however, that the vast majority of TRT patients that attribute their low Testosterone to andropause is the result of Leydig cell aging rather than reduced output of LH secretion from the pituitary gland1. Therefore, in such cases, the only possible option for their treatment of low Testosterone is to engage in the use of exogenous Testosterone for the purpose of Testosterone replacement therapy. For those individuals suffering from low Testosterone as the result of reduced LH and FSH output, which is for example very typical of ASIH (Anabolic Steroid Induced Hypogonadism) sufferers, the first line of treatment would typically be the attempt to recover the HPTA through Testosterone stimulating compounds. It is a very well-known fact that there is a vast suppression of serum gonadotropins, and subsequently Testosterone levels, following anabolic steroid use that will normally continue for an indefinite period following the termination of anabolic steroids[13] [14] [15]. The problem with administering HCG alone or engaging sufferers of this type of low Testosterone in TRT is that both of these treatments are indefinite in nature – they require consistent lifelong administration, and do not lead to the body’s endogenous production continuing on its own without outside assistance.
The use of aromatase inhibitors (AIs), Selective Estrogen Receptor Modulators (SERMs) and HCG all together in a combined treatment is normally utilized to attempt to restore HPTA function without the requirement for lifelong TRT administration. Following this, if these therapies do not succeed, then TRT is typically the second line of treatment. Aromatase inhibitors in particular have been shown in many studies to elevate the gonadotropins LH and FSH, and consequently, total serum Testosterone levels[16]. As far as SERMs are concerned, they are in fact used as a frequent treatment for HPTA recovery within medicine due to their Estrogen antagonistic actions on the pituitary gland, which also consequently leads to an increase in Testosterone production by the Leydig cells of the testes[17] [18] [19] [20] [21]. Although the standard treatment for low Testosterone is that of TRT, more and more medical professionals are continually adopting the use of endogenous Testosterone stimulating agents as previously described in order to treat sufferers of low Testosterone that do not exhibit Leydig cell nonfunctioning due to the aging process[22]
Medical References:
[1] Aging and declining testosterone: past, present, and hopes for the future. Zirkin BR, Tenover JL. J Androl. 2012 Nov-Dec;33(6):1111-8. doi: 10.2164/jandrol.112.017160. Epub 2012 Aug 9.
[2] Do androgens play any role in the physical frailty of ageing men? O’Connell MD, Tajar A, Roberts SA, Wu FC. Int J Androl. 2011 Jun;34(3):195-211. doi: 10.1111/j.1365-2605.2010.01093.x. Epub 2010 Aug 17.
[3] “Unexplained anemia in the elderly”. Makipour S, Kanapuru B, Ershler WB (October 2008). Semin. Hematol. 45 (4): 250–4. doi:10.1053/j.seminhematol.2008.06.003. PMC 2586804. PMID 18809095.
[4] “Testosterone replacement therapy in hypogonadal men: assessing benefits, risks, and best practices”. Miner M, Canty DJ, Shabsigh R (September 2008). Postgrad Med 120 (3): 130–53. doi:10.3810/pgm.2008.09.1914. PMID 18824832.
[5] “Trends and determinants of prescription medication use for treatment of osteoporosis”. Farley JF, Blalock SJ (July 2009). Am J Health Syst Pharm 66 (13): 1191–201. doi:10.2146/ajhp080248. PMID 19535658.
[6] Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Boyanov MA, Boneva Z, Christov VG. Aging Male. 2003 Mar;6(1):1-7. PMID: 12809074
[7] “The dark side of testosterone deficiency: II. Type 2 diabetes and insulin resistance”. Traish AM, Saad F, Guay A (2009). J. Androl. 30 (1): 23–32. doi:10.2164/jandrol.108.005751. PMID 18772488.
[8] “Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure a double-blind, placebo-controlled, randomized study”. Caminiti G, Volterrani M, Iellamo F, et al. (September 2009). J. Am. Coll. Cardiol. 54 (10): 919–27. doi:10.1016/j.jacc.2009.04.078. PMID 19712802.
[9] Depression is correlated with the psychological and physical aspects of sexual dysfunction in men. Pastuszak AW, Badhiwala N, Lipshultz LI, Khera M. Int J Impot Res. 2013 Mar 7. doi: 10.1038/ijir.2013.4.
[10] “Testosterone effects on cognition in health and disease”. Cherrier MM (2009). Front Horm Res 37: 150–62. doi:10.1159/000176051. PMID 19011295.
[11] Androgen Deficiency in the Aging Male. Carruthers, Malcolm (2004). London: Taylor & Francis Group. ISBN 1-84214-032-9.
[12] Is Testosterone Treatment Good for the Prostate? Study of Safety during Long-Term Treatment. Feneley MR, Carruthers M. J Sex Med. 2012 Jun 6. doi: 10.1111/j.1743-6109.2012.02808.x.
[13] Effect of anabolic treatment on the serum levels of gonadotropins, testosterone, prolactin, thyroid hormones and myoglobin of male athletes under physical training. Clerico A, Ferdeghini M, Palombo C, et al. J Nucl Med Allied Sci 1981;25:79–88.
[14] Response of serum testosterone and its precursor steroids, SHBG and CBG to anabolic steroid and testosterone selfadministration in man. Ruokonen A, Alen M, Bolton N, Vihko R. J Steroid Biochem 1985;23:33–8.
[15] Physical health and fitness of an elite bodybuilder during 1 year of self-administration of testosterone and anabolic steroids: a case study. Alen M, Hakkinen K. Int J Sports Med 1985;6:24–9.
[16] Treatment of male infertility secondary to morbid obesity. Roth MY, Amory JK, Page ST. Nat Clin Pract Endocrinol Metab 2008;4(7):415–9.
[17] Pulsatile patterns of gonadotropins and testosterone in man: the effects of clomiphene, with and without testosterone. Naftolin F, Judd HL, Yen SSC. J Clin Endocrinol Metab 1973;36:285–8.
[18] Evidence for a role of endogenous estrogen in the hypothalamic control of gonadotropin secretion in men. Winters SJ, Troen P. J Clin Endocrinol Metab 1985;61:842–5.
[19] Studies on the role of sex steroids in the feedback control of gonadotropin concentrations in men. II. Use of the estrogen antagonist, clomiphene citrate. Winters SJ, Janick JJ, Loriaux DL, Sherins RJ. J Clin Endocrinol Metab 1979;48:222–7.
[20] Short- and long-term effects of clomiphene citrate on the pituitary–testicular axis. Santen RJ, Leonard JM, Sherins RJ, Gandy HM, Paulsen CA. J Clin Endocrinol Metab 1971;33:970–6.
[21] Estrogens in the feedback regulation of gonadotropin secretion in men: effects of administration of estrogen to agonadal subjects and the antiestrogen tamoxifen and the aromatase inhibitor d1-testolactone to eugonadal subjects. Gooren LJ, Van der Veen EA, van Kessel H, Harmsen-Louman W. Andrologia 1984;16:568–77.