Winstrol History and Overview
Winstrol is the trade name and brand name for the anabolic steroid more formally and properly known as Stanozolol. It exists in both an oral and injectable format, and it sits on the third place pedestal among the top 3 anabolic steroids most widely used and most popular among bodybuilders and athletes. It is superseded only by Deca-Durabolin (Nandrolone Decanoate) in second place, and Dianabol (Methandrostenolone) in first place. Winstrol is probably most famous and well-known for its alleged use by Canadian Olympic sprinter Ben Johnson in the 1988 Seoul Summer Olympic games where he tested positive for it. However, its origins begin in 1959 when its details were first released in the medical and scientific community[1]. It was Winthrop Laboratories in the UK that marketed Stanozolol on the prescription drug market, and Sterling in the USA ended up buying the patent in 1961 for the US market where it was branded as Winstrol[2].
Winstrol was approved for an extensive list of medical treatments following its release onto the prescription drug market, ranging from tissue and muscle wasting diseases to an Oesteoporosis drug, as well as in the treatment of burn victims and children with stunted growth. Winstrol was not very effective as a treatment for anemia, however[3]. Winstrol’s mechanism of action is quite brilliant in several different ways. Its primary operation is through binding with the androgen receptor rather than non-receptor mediated activity[4], which is common with certain other anabolic steroids such as Dianabol or Anadrol. Winstrol also has a low affinity for binding with the Glucocorticoid-binding sites in addition to some activity that is independent of androgen receptors[5][6][7][8]. Winstrol also possesses a very low degree of Progestogenic activity in the body[8].
An impressive and somewhat unique function that Winstrol exhibits is its ability to significantly lower SHBG (Sex Hormone Binding Globulin) levels in the body, allowing freer Testosterone and other anabolic hormones to be able to exert anabolic activity in muscle tissues[10]. It exhibits exceptional capability and a high degree of stimulating and facilitating protein synthesis[11][12]. Winstrol has also been observed to possess a capability of boosting collagen synthesis in the body[13].
Chemical Characteristics of Winstrol
Winstrol’s chemical structure differs very significantly from every other anabolic steroid. It is, however, a derivative of Dihydrotestosterone (DHT) where it contains a 3-2 Pyrazol group attached to the first cycloalkane ring (known as the A-ring) of the anabolic steroid structure. This is actually very noticeable when a picture of the chemical structure of Winstrol is laid side-by-side with its progenitor hormone DHT, even to an individual unfamiliar with chemistry. The Pyrazol group’s attachment to the A-ring actually replaces the 3-keto group that normally sits in the same location. Specifically, this major modification classifies Winstrol as what is known as a Heterocyclic steroid.
This Pyrazol group, which is a functional group, is actually responsible for Winstrol’s stronger binding affinity for the androgen receptor in muscle tissue. As a DHT-derivative with modifications that separate its distinction with DHT, Winstrol is actually active in muscle tissue to a far greater degree than DHT itself is. Unfortunately, DHT itself is rendered inactive almost immediately by two enzymes upon its entrance into muscle tissue. Winstrol’s modifications allow it to effectively avoid this problem. All anabolic steroids that belong to the family of DHT-derivatives (such as Winstrol, Anavar, Primobolan, Masteron and several others) contain modifications to their chemical structures that grant them significant activity and effectiveness within muscle tissue, where DHT itself unmodified would never survive metabolism there. The Pyrazol structure also grants Winstrol a significant shift in its anabolic and androgenic strengths to favor more of a stronger anabolic strength while greatly reducing its androgenic strength. This is what grants Winstrol with an incredibly strong disassociation of anabolic to androgenic effects.
Winstrol also possesses a methyl group attached to the 17th carbon (known as C17 Alpha Alkylation), which is the chemical structural modification that allows the anabolic steroid to survive the first pass through the liver when ingested orally, and allows the anabolic steroid to become further resistant to hepatic metabolism.
Properties of Winstrol (Stanozolol)
Studies have demonstrated that Winstrol’s main mechanism of action is that of binding with cellular androgen receptors as opposed to non-receptor mediated activity (such as those possessed by Dianabol or Anadrol). It is also believed that Winstrol also possesses some very small measurable form of anti-Progestogenic properties in regards to the Progesterone receptor, although this is not fully understood. In addition to some small antagonistic effects on the Progesterone receptor, it has been found that Winstrol also possesses low affinity for Glucocorticoid-binding site interactions, as well as activity that is independent of Androgen receptors, Progesterone receptors, and Glucocorticoid receptors. Winstrol has not been found to have any notable Progestogenic activity in the body as well.
Winstrol possesses a very high binding affinity for SHBG (Sex Hormone Binding Globulin), therefore granting far more of Winstrol (as well as other anabolic steroids that may be stacked alongside it, such as Testosterone) to freedom in the bloodstream in doing its job of signaling muscle growth. SHBG is a protein that attaches and binds to other sex hormones (Testosterone, Estrogen, or any synthetic anabolic steroid) and renders them useless as long as SHBG is bound to that hormone. Effectively, SHBG places ‘handcuffs’ on any hormone it binds to and prevents it from doing its job. Winstrol has also demonstrated to not only prevent SHBG from binding with other anabolic steroids, but it has also demonstrated strong suppression of SHBG production in the body. For example, one particular study conducted on 25 male test subjects where Winstrol was administered orally resulted in a 48.4% drop in SHBG levels following just 3 days of Winstrol administration.
With Winstrol being a DHT-derivative, it holds the advantage that is generally associated with DHT and all other DHT-derivatives: it is unable to bind with the aromatase enzyme, which results in no possible Estrogen conversion. Resulting from this is an avoidance of the Estrogen-related side effects of water retention (and the associated risks of elevated blood pressure), as well as other Estrogen-related side effects. Being a DHT-derivative, it is also unable to interact with the 5-alpha reductase enzyme, which is the enzyme responsible for the conversion of Testosterone into Dihydrotestosterone. As Winstrol is already a modified form of DHT, this cannot possibly occur.
Winstrol exhibits a longer half-life as a result of its structural modifications, enabling the injectable format of Winstrol to possess a half-life of approximately 24 hours, and 9 hours for the oral preparation of Winstrol. In relation to Testosterone, Winstrol holds an androgenic strength rating of 30 with an anabolic strength rating of 320, which is quite significant considering this means Winstrol is slightly over three times the anabolic strength of Testosterone. In order for any individual to understand the meaning of these numbers and ratings, it must be understood that the base reference measurement for these strength ratings is the number one anabolic steroid Testosterone. Testosterone is utilized as the measuring stick or the measuring bar whereby all other anabolic steroids are referenced with and compared to (much like the celcius scale of temperature measurement where the freezing point and boiling points of water is used as the baseline measurement for temperature). Upon understanding this, any individual can easily observe how Winstrol possesses an anabolic strength of three times Testosterone (Testosterone’s anabolic and androgenic ratings are both respectively 100). Percentage-wise, it could be described that Winstrol is 320% more anabolic than Testosterone, and it is 30% less androgenic than Testosterone.
An important fact that must be reminded to the reader is the fact that both the injectable and oral preparations of Winstrol possess the exact same chemical structure. This is unlike nearly all other anabolic steroids, where oral preparations are always C17-alpha alkylated, and injectable preparations are absent of this methylation (and often injectable compounds are also esterified to modulate the release rate and half-life). This is not so with Winstrol, where the oral and injectable preparations are exactly 100% identical to each other. This presents some concerns that the reader must be aware of: The result is a greater amount of hepatotoxicity (liver toxicity), and because both the injectable and oral preparations both possess the hepatotoxic modification of C17-alpha alkylation, they both will place an almost equal level of hepatotoxic strain on the liver. However, the injectable preparation avoids the first-pass through the liver, which allows it to be slightly less hepatotoxic than the oral Winstrol preparation – but hepatotoxic nevertheless, and its duration of use must also have limitations placed on it.
Winstrol Side Effects
The primary areas of concern when it comes to Winstrol side effects are, simply put: hepatic (liver) issues relating to hepatotoxicity, HPTA (Hypothalamic Pituitary Testicular Axis) disruption, and negative cardiovascular system impacts.
Because Winstrol is a DHT-derived anabolic steroid, it cannot convert into Estrogen at any dose. This means that no Estrogenic side effects should be experienced as a result of Winstrol use alone. Therefore, side effects such as gynecomastia, water retention and bloating, or other side effects as a result of Estrogen buildup are completely nonexistent here.
Although Winstrol does possess a weaker androgenic strength rating than Testosterone does, androgenic side effects are still a potential risk, though much lower, and may only effect users significantly if they are especially sensitive to androgens. Androgenic side effects can include increased oily skin and acne, hair growth on the body, MPB (male pattern baldness), and BPH (Benign Prostatic Hypertrophy). The use of a 5-alpha reductase (5AR) inhibitor, such as Finasteride, Dutasteride, Proscar, or Propecia will be completely ineffective here, as Winstrol does not convert into DHT.
One area of prominent concern is that of HPTA suppression, which is a given with the use of any anabolic steroid. Studies have demonstrated that with even as low as 10mg/day of Winstrol, subjects experienced a 55% decrease in endogenous Testosterone production after only 14 days[14]. Hepatotoxicity (liver toxicity) is also one effect that one should be conscious of with Winstrol, especially with use of the oral variant[15]. The injectable preparation of Winstrol has also raised concerns in studies, where severe hepatotoxicity was eventually experienced by otherwise healthy individuals[16]. It is therefore recommended to utilize Winstrol, especially the oral variant, for periods of no longer than 6 – 8 weeks at a time.
Cardiovascular health concerns are particularly major when it comes to Winstrol side effects. First and foremost, Winstrol is notorious for producing very severe and dangerous negative alterations in blood cholesterol levels, even with miniscule dosages of the oral format (as low as 6mg/day)[17]. Even the injectable preparation of Stanozolol has demonstrated very negative implications on blood cholesterol[18]. To add further concern to Winstrol’s cardiovascular effects, evidence suggests that it can also stimulate cardiac hypertrophy at even small dosages[19].
Winstrol Cycles and Uses
Winstrol cycles are normally intended for the purpose of fat loss, cutting, and pre-contest preparation where the end goal is to achieve very low body fat levels and a high level of definition in the physique. There are those who claim it can also be utilized for bulking, mass, and strength gaining, but there are other anabolic steroids that are generally cheaper and better suited for these purposes than Winstrol. It is generally accepted that Winstrol cycles should be reserved for cutting and fat loss.
As such, Winstrol cycles normally include a base compound of similar use, normally something such as Testosterone Propionate for an 8 – 10 week cycle. Intermediate and advanced Winstrol cycles can also involve three compounds in total, normally a stack such as Testosterone Propionate, Trenbolone Acetate, and Winstrol (either the oral or injectable preparation will do).
It is important to also note that because of the hepatotoxicity differences between the oral and injectable Winstrol preparations, it is possible to run Winstrol cycles longer with the injectable than the oral (8 – 10 weeks for the injectable and 6 – 8 weeks for the oral respectively).
Winstrol Dosages and Administration
Winstrol is a very versatile anabolic steroid, and its dosages vary extensively (especially between the oral and injectable variants).
Winstrol dosages in the medical arena originally called for a 6mg per day dosage, ideally split up throughout the day (e.g. a 2mg tablet administered 3 times per day). The injectable Winstrol as a medicine was typically prescribed at a dosage of 50mg only once every two to three weeks. However, for the purposes of athletics and performance enhancement, these medical dosages and frequencies for Winstrol do not help at all.
Winstrol Depot ZAMBON 50mg/ml Amps
For athletics, physique, and strength, general Winstrol dosages range (with the injectable) around 50 – 100mg administered every other day, equating to around 200 – 400mg per week. In terms of oral Winstrol, this averages to around 60mg per day, and lower dosages of Winstrol (such as 25 – 50mg per day) are said to work well for all groups of users and athletes. Because Winstrol is not an incredibly strong anabolic steroid meant for bulking and mass gaining, there is no need to rise dosages to extreme or dangerous levels, and for the purposes of aiding definition and fat loss, the aforementioned dosages here are good ranges.
For females, 5 – 10mg per day of the oral preparation is known to be pretty common among female bodybuilders and athletes that want to avoid any risk of virilization or other side effects. Although injectable Winstrol is not very common among female athletes and bodybuilders, 15mg injected every other day (for an approximate total of 60mg per week) is a good recommendation.
How to Buy Winstrol
It goes without saying that a compound like Winstrol, which holds third in the top 3 ranking for most widely used and most popular anabolic steroid, it is a very common compound on the market. Those wishing to buy Winstrol will have no problems locating it anywhere in the world from any source. As should be common knowledge by the reader at this point, Winstrol is commercially available in two formats: injectable and oral. The injectable is normally in the form of a water-based suspension, but some underground labs (UGLs) have discovered methods of manufacturing it into an oil-based injectable. These are known to be exclusively underground products at this time without question, as oil-based injectable Winstrol is not yet an official pharmaceutical grade product sold as a medicine on the prescription market under any circumstances.
Many different source types exist, ranging from internet sources and websites to in-person dealers, which all affect the prices that one can buy Winstrol for on the black market. Additionally, some of these sources may impose certain buying conditions (such as minimum order limits) that affect prices heavily as well. In any case, oral Winstrol can be found priced in the range of $1.50 – $3.00 per 50mg tablet. Keep in mind as well that Winstrol tablets can also be found in other concentrations (such as 5, 10, and 25mg tablets, etc.). Injectable Winstrol can be found on the market anywhere from $60 – $120 for a 10ml vial with a standard concentration of 50mg/ml. Although 50mg/ml is a pharmaceutical standard for injectable Winstrol, many underground labs are now manufacturing higher concentrations (such as 100mg/ml) and have been doing so for quite some time now. It is important to note, however, that such products with higher concentrations are exclusively underground products – no human grade pharmaceutical products are known to hold concentrations of greater than 50mg/ml.
Winstrol Information:
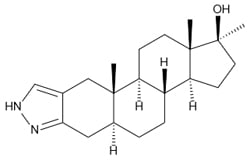 Winstrol (AKA Stanozolol)
Winstrol (AKA Stanozolol)
Chemical Name: 17β-Hydroxy-17-methyl-5alpha-androstano[3,2-c]pyrazole
Molecular Weight: 328.49 g/mol
Formula: C21H32N2O
Original Manufacturer: Winthrop Laboratories
Half Life: 9 hours (oral), 24 hours (injectable)
Detection Time: 2 months
Anabolic Rating: 320
Androgenic Rating: 30
Winstrol References:
- Clinton R. O. et al. J. Amer chem. Soc. 81 (1959):1513.
- S. Patent # 3,030,358.
- Trop Doct. 2004 Jul;34(3):149-52.
- Anabolic-androgenic steroid interaction with rat androgen receptor in vivo and in vitro: a comparative study. Feldkoren Bl, Andersson S. J Steroid Biochem Mol Biol 2005 Apr;94(5):481-7. Epub 2005 Mar 17.
- The differential effects of stanozolol on human skin and synovial fibroblasts in vitro: DNA synthesis and receptor binding. Ellis AJ, Cawston TE, Mackie EJ. Agents Actions. 1994 Mar;41(1-2):37-43.
- Identification of a specific binding site for the anabolic steroid stanozolol in male rat liver microsomes. Boada LD, Fernandez L et al. J Pharmacol Exp Ther 1996 Dec;279(3):1123-9.
- Stanozolol and danazol, unlike natural androgens, interact with the low affinity glucocorticoid-binding sites from male rat liver microsomes. Fernandez L, Chirino R, Boada LD, Navarro D, Cabrera N, del Rio L, Diaz-Chico BN. Endocrinology. 1994 Mar;134(3):1401-8.
- Di Yi Jun Yi Da Xue Xue Bao. 2003 Nov;23(11):1117-20.
- Desaulles P.A. et al. Helv. Med Acta 27 (1960), 479.
- Sex hormone-binding globulin response to the anabolic steroid stanozolol: Evidence for its suitability as a Biological androgen sensitivity test. G Sinnecker, S Kohler. Journal of Clin Endo Metab. 68:1195, 1989.
- Can J Vet Res. 2000 Oct;64(4):246-8.
- J Am Vet Med Assoc. 1997 Sep 15;211(6):719-22.
- J Invest Dermatol. 1998 Dec;111(6):1193-7.
- Alteration of hormone levels in normal males given the anabolic steroid stanozolol. Small M, Beastall GH, Semple CG, Cowan RA, Forbes CD, Clin Endocrinol (Ocf). 1984 Jul;21(1):49-55.
- The influence of 6 months of oral anabolic steroids on body mass and respiratory muscles in undernourished COPD patients. Ivone Martins Ferreira, leda Verreschi et al. CHEST 114 (1) July 1998 19-28.
- Androgenic/Anabolic steroid-induced toxic hepatitis. Stimac D, Milic, S Dintijana RD, Kovac D, Ristic S. J Clin Gastroenterol. 2002 Oct;35(4):350-2.
- Contrasting effects of testosterone and stanozolol on serum lipoprotein levels. Thompson TD, Cullinane EM, Sady SP, Chenevert C, Saritelli AL, Sady MA, Herbert PN. JAMA. 1989 Feb 24;261(8):1165-8.
- The effect of intramuscular stanozolol on fibrinolysis and blood lipids. Small M, McArdie BM, Lowe GD, Forbes CD, Prentice CR, Thromb Res. 1982 Oct 1;28(1):27-36.
- J Steroid Biochem Mol Biol. 2005 Jan;93(1):43-8. Epub 2005 Jan 25.





