Clomid As Hormone Replacement Therapy (HRT)
Blog Entry #64
By Admin – Steroidal.com
Clomid or clomiphene citrate, is a selective estrogen receptor modulator (SERM) and is used medically for a variety of treatments. Clomid first came to the market in the 1970s for the treatment of female infertility. Clomid is a mixed agonist and antagonist of the estrogen receptor. This means it acts as estrogen in some tissues, whilst in others it blocks estrogen. This is comparable with all SERMs as some are better than others at raising testosterone, like Clomid, and others are potent at blocking the estrogen receptor in breast tissue, such as Rolaxifene.
As time has passed and more is understood about Clomid and its effects on females and males, it’s now used as a treatment for male infertility, and in some countries as a hormone replacement therapy (HRT). Clomid being used as HRT medication is what we’re going to discuss today. Clomid and other SERMs raise testosterone levels in males due to their action of blocking the estrogen receptor in the brain. More can be read about this in our post cycle therapy (PCT) article.
Hormone replacement treatments for males include; testosterone gels, creams, sprays, pellets, to oral testosterone preparations and finally, injectable testosterones. Regardless of the delivery method, the treatment exists to replace the already low testosterone level due to age, disease, steroid use, genetics, and infertility or for a better quality of life. However, because of the method of action of SERMs on males, we can instead attempt to raise endogenous testosterone output, but this would only work if the subject’s testosterone level is already low and not zero per se.
Secondary hypogonadism is the failure of the hypothalamus and pituitary producing natural hormones to stimulate testosterone production by the testes. This can be treated by Clomid use, and we see this when coming off of anabolic steroids during PCT. Primary hypogonadism is when the testes fail and need to be treated with testosterone replacement therapy.
The study we’re going to look at today was done in 2011 at the Memorial Sloan-Kettering Cancer Center in New York. Clomid was given to 86 men different doses of Clomid as an alternate hormone replacement therapy. The men were aged between 22 and 37 years old and given either 25mg every other day of Clomid or 50mg every other day for 19 months. Seventy per cent took the lower dose of 25mg every other day and the rest took the higher Clomid dosage. Compared to PCT protocols, this is a low dose as these doses are used every day, not every other day, but PCTs also last around 4-6 weeks and not 19 months.
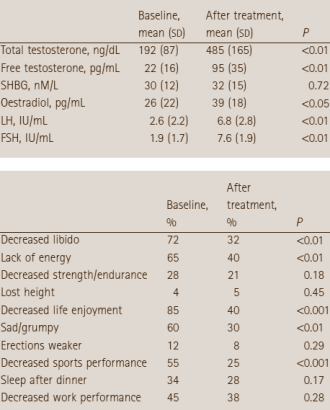
The table above shows the average hormone increases of the men given Clomid. As you can see, total testosterone (TT), leutinizing hormone (LH) more than doubles. Whilst follicle stimulating hormone (FSH) increases by more than 300%. These are favourable results even compared to injecting a synthetic version of testosterone. The results of a pre and post treatment questionnaire are also shown, which indicate less of the males had a loss of labido after treatment.
“Clomiphene citrate is an effective and safe alternative to testosterone supplementation therapy in hypogonadal men”, the endocrinologists conclude. “Clomiphene citrate therapy has a role to play in the testosterone deficient man and should be incorporated into the clinician-patient discussion.”
Limitations are that Clomid can bring its own side effects. Low of libido, mood swings, and vision disturbances are evident in some users, but these seem to become apparent in larger doses exceeding 100mg per day. We would suggest some sort of alternative hormone replacement therapy similar to the above protocol, with the addition of herbal products to enhance libido and possible testosterone production naturally. If these protocols fail, hormone replacement therapy in the format of injectable estered testosterone would be a viable option.
Source:
BJU Int. 2011 Nov 1. doi: 10.1111/j.1464-410X.2011.10702.x. [Epub ahead of print].